Latest Galary
Fuel Intro
Yes, we are talking of Anhydrous Ammonia Gas (AAG) you must had have smelled it if you have ever been to a collage chemistry lab, or this is the same gas that makes you trouble in the public urinals of non hygienic toilets. Because it does not occur naturally in its pure form on our planet and must be manufactured, we can consider it an energy carrier rather than an energy source. Be that as it may, we will consider here Anhydrous Ammonia as an ICE fuel.
As for fuel properties,let's compare some relevant fuels
| Property | AAG | Hydro-gen | LPG / Propane | CNG / Methane | Petrol | Diesel |
|---|---|---|---|---|---|---|
| Energy Density LHV (MJ/liter) | 14.1 | 8.6 (liquid) | 26.8 (liquid) | 25.3 @ 2400 psi |
34.8 | 38.6 |
| Weight per Liter | 682 gm | 71 gm | 507.7 gm | 460 gm | 784 gm | 850 gm |
| EnergyDensity MJ /Kg | 20.67 | 121 | 46 | 55 | 44.4 | 45.4 |
| OctaneNumber | 130+ | 130+ | 104 | 105 - 122 | 87 - 93 | 15-25 |
| Auto ignition Temp(°C) | 651 | 571 | 480 | 500 | 246 | 210 |
| Flash Point (°C) | 11 | -253 | -- | -188 | <-40 | >62 |
| Boiling Point (°C) | -33 | -253 | -- | -162 | 126 | 287 |
| Critical Temp- erature (°C) | 132 | -240 | 96.6 | -83 | -- | -- |
| Critical pressure (°C) | 112 | 12.98 | 42.5 bar | 45.96 bar | -- | -- |
| Achieved ICE efficiency | ~50% | 25-35% | 20-25% | 20-25% | 18-20% | 20-25% |
| Minimum Ignition Energy (MJ) | 680 | 0.011 - 0.017 | n/a | 0.28 - 0.3 | 0.8 | n/a |
| CO2 emission/Kg Consumption | 0.00 | 0.00 | 3.00 Kg | 2.75 Kg | 3.087 Kg | 3.155 KG |
NOX SOX & Other pollution |
NO | NO | YES | YES | YES | YES |
It is clear that we have only tow fuels which can deliver us green pollution free energy and also these are renewable, one is hydrogen and second is the AAG.
Hydrogen density and higher heating value (HHV) in different fuels
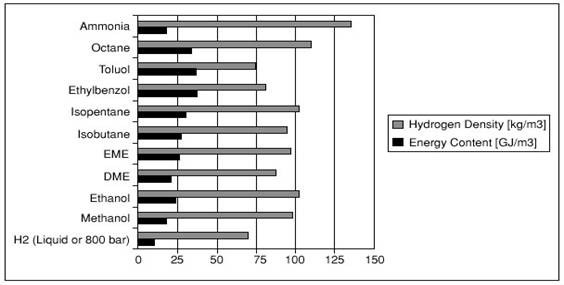
DME = dimethyl ether and EME = ethyl methyl ether.
AAG: The Fuel for the Hydrogen Economy
Why?
- Our research has shown that there are clear advantages to using AAG instead of hydrogen as fuel for automobiles. The ammonia molecule has 3 hydrogen atoms and 1 nitrogen atom. It contains absolutely no carbon whatsoever. It is easy to make on an industrial scale by means of the Haber process and it is easy to use as a fuel. It can be reformed back into nitrogen and hydrogen so that the latter can be used in fuel cells although there are fuel cells that can operate directly from Ammonia. And it can be burned directly in an internal combustion engine albeit with some modification, resulting in an exhaust of water and nitrogen. Ammonia is therefore, a good 'carrier' of hydrogen.
- Hydrogen carriers are of interest because hydrogen on its own is very difficult to store and by inference, to transport. By weight, hydrogen has far higher energy content than any other fuel. But by volume it has by far the lowest. Roughly speaking, 1Kg of hydrogen contains about as much energy as 4 liters of gasoline, but at normal atmospheric pressure, occupies a staggering 11 cubic meters! To be as energy dense by volume as gasoline we would need to compress the hydrogen to something approaching 3000 atmospheres pressure. Even with a more achievable 300 atmospheres a hydrogen tank would be 10 times the size of a gasoline tank containing the same amount of energy.
- And in addition tanks capable of withstanding that sort of pressure are very heavy and expensive. Typically a modern tank made of carbon fiber composites, would weigh some 15 to 20 times the weight of the hydrogen it contains and weigh more than the equivalent gasoline tank. This puts paid to hydrogen's advantage of being the most energy dense fuel by weight.
- And it gets worse. Hydrogen has the smallest atomic size of all elements and as a result, has a nasty habit of seeping into and through the walls of the tank, weakening their structure and posing a significant risk of structural failure. Clearly with 300 atmospheres of highly flammable gas involved, such an outcome is unwelcome. Tanks can be lined with a material less susceptible to hydrogen infusion and this certainly helps but only up to a point. Compressed hydrogen tanks will likely need regular inspection and are likely to have limited lifetimes.
- Some car manufacturers have experimented with liquid hydrogen stored in a Dewar. But it takes a lot of energy, about 40 percent of the fuel's energy content, to refrigerate hydrogen to the -253°C needed to liquefy it. And of course, the hydrogen in the tank won't stay cold and liquid all by itself. While Dewars are very effective at keeping out the heat, they are not perfect and heat from the outside slowly creeps in causing the hydrogen to steadily boil off. It either has to be vented which adds to the wastage or the tank has to incorporate a cryostat which is a very expensive extra. In our view, liquid hydrogen for applications other than taking men to the moon is completely impractical.
- The problems posed by compression are less than those posed by liquidation making compression a possible albeit expensive option for road transport where weight was not critical. The tank size would be mitigated if we use fuel cells instead of an internal combustion engine as the greater efficiency would require less fuel for the same distance. And we could accept a shorter range. But compression is a much less plausible for aircraft. Implementation of the hydrogen economy directly by using hydrogen is not really a practical proposition.
These are the conditions and problems that compel us to opt for a hydrogen carrier.
Advantages of AAG over Hydrogen
- AAG suffers from none of the storage and transportation problems posed by hydrogen. Instead of needing tanks capable of withstanding enormous pressure, ammonia is a liquid at ordinary temperatures when pressurized to a mere 8 atmospheres and there is no tendency for ammonia to pervade the walls of the tank. This is comparable to LPG and means we can use cheap, reliable tanks that last and do not require regular intensive inspection.
- Conversion of hydrogen into ammonia
- 1 kg H2 yield 5.66 kg ammonia that is to say in energy terms 121 MJ energy of hydrogen can be converted into 117 MJ energy of ammonia which results in a fuel that is cheap and easy to handle, transport, and store.
- The hydrogen economy was never going to come for free but the drawbacks of ammonia are in our view an acceptable price to pay for carbon neutrality. And compared to compressed hydrogen or the even worse liquid hydrogen, ammonia is a dream./li>
- The ammonia fueled IC Engines display 50% Fuel Efficiency which is attractive and the greatest of all fuels including Hydrogen itself.
- AAG can be burned in an IC Engine and this allows us to take advantage of the huge legacy of pre-exiting cars with IC Engines and the manufacturing infrastructure that builds them. Ideally the IC Engine would be built from scratch to a design specifically optimized for ammonia but existing IC engines can be converted with satisfactory results.
- Most important is that it will cost cheaper in comparison to any fossil of bio fuel thus reduces the running cost of the vehicle or related system.
Hazards of the AAG
- In normal atmospheric conditions it is essentially non inflammable thus in case of leakage it will not catch fire.
- Ammonia has a very nasty smell so small leaks (5 ppm) are easily detected, but larger leaks are a serious threat to health. Concentrations of 5,000 ppm concentration in air may be harmful and 10000 ppm concentration may be dangerous if inhaled. It sounds scary but nor would you want to be to close to a petrol tank that was about to be involved in an accident and rupture? The reason we never think of this is because decades have passed since being in a car accident commonly resulted in people getting cooked. Modern cars are designed to prevent tank rupture even in the most serious accidents and the strategy works.
- It is actually more meaningful to compare ammonia not to petrol but to LPG as both are liquefied gasses under pressure. Tank rupture is clearly seen as being a very small risk by authorities charged with ensuring safety. If this were not the case there would not be LPG and CNG vehicles.
- In the event of a rupture, LPG is not going to poison anyone as ammonia could, but on the other hand, ammonia is much less likely to turn people into kebabs. With an ignition temperature in the region of 650°C, ammonia outside an engine is essentially inflammable.
- We are storing Hydrogen at 5000 psi CNG at 3000 psi and LPG at 250 psi then why we can not store and handle AAG at 150 psi and insure safety. As we are already widely using the same in fertilizer and refrigerating industry since about last eighty years. And I don’t know any ammonia accident took place in the country.
- Pollution free zero emission Hydrogen Economy will never come for free. We are well acquainted with ammonia and its pros and cons are well known to us. We fell in trouble when we are not aware of the nature of risk but if we are aware of the risks associated with the substance then we can ensure safe and profitable utilization and handling of the same. And we will do the same.









