Latest Galary
Ammonia as Chemical
| Property | Value | |||||
|---|---|---|---|---|---|---|
| Molar mass | 17.031 g/mol | |||||
| Appearance | Colorless gas with strong pungent odor | |||||
| Density | 0.86 kg/m3 (1.013 bar at boiling point) 0.73 kg/m3 (1.013 bar at 15 °C) 681.9 kg/m3 at −33.3 °C (liquid) 820 kg/m3 at -80 °C (crystal solid) 817 kg/m3 at -80 °C (transparent solid) |
|||||
| Melting point | −77.73 °C (195.42 K) | |||||
| Boiling point | −33.34 °C (239.81 K) | |||||
| Solubility in water | 1176 g/Liter (0 °C) 702 g/Liter (20 °C) 88 g/Liter (100 °C) |
|||||
| Acidity (pKa) | 9.245 | |||||
| Basicity (pKb)) | 4.75 | |||||
| Critical temp | 132.4 0C | |||||
| Critical pressure | 112.bar / 1636psi / 115 kg | |||||
Ammonia is a colorless gas with a characteristic pungent smell. It is lighter than air, its density being 0.589 times that of air. It is easily liquefied due to the strong hydrogen bonding between molecules; the liquid boils at −33.3 °C, and solidifies at −77.7 °C to white crystals. Liquid ammonia has a very high standard enthalpy change of vaporization (23.35 kJ/mol, therefore it is used in laboratories in non-insulated vessels without additional refrigeration.
It is miscible with water. Ammonia in an aqueous solution can be expelled by boiling. The aqueous solution of ammonia is basic. The maximum concentration of ammonia in water (a saturated solution) has a density of 0.880 g /cm3 and is often known as '.880 Ammonia'. Ammonia does not burn readily or sustain combustion, except under narrow fuel-to-air mixtures of 15-25% air. When mixed with oxygen, it burns with a pale yellowish-green flame. At high temperature and in the presence of a suitable catalyst, ammonia is decomposed into its constituent elements. Ignition occurs when chlorine is passed into ammonia, forming nitrogen and hydrogen chloride; if ammonia is present in excess, then the highly explosive nitrogen trichloride (NCl3) is also formed.
The ammonia molecule readily undergoes nitrogen inversion at room temperature; a useful analogy is an umbrella turning itself inside out in a strong wind. The energy barrier to this inversion is 24.7 kJ/mol, and the resonance frequency is 23.79 GHz, corresponding to microwave radiation of a wavelength of 1.260 cm (0 in). The absorption at this frequency was the first microwave spectrum to be observed.
Physical properties
- Ammonia Melting point : -78oC
- Ammonia Latent heat of fusion (1,013 bar, at triple point) : 331.37 kJ/kg
- Ammonia Liquid Density (1.013 bar at boiling point) : 682 kg/m3 (250 K : 669 kg/m3 ) (300 K : 600 kg/m3) (400 K : 346 kg/m3 )
- Ammonia Liquid Specific Heat Capacity (cp) (250 K : 4.52 kJ/kg.K) (300 K : 4.75 kJ/kg.K) (400 K : 6.91 kJ/kg.K)
- Ammonia Liquid/gas equivalent (1.013 bar and 15oC (59oF)): 947 vol/vol
- Ammonia Liquid Dynamic Viscosity (250K : 245 106 Ns/m2) (300K : 141 106 Ns/m2) (400K : 38 106 Ns/m2)
- Ammonia Liquid Thermal Conductivity (250 K : 592 106 kW/m.K) (300 K : 477 106 kW/m.K) (400 K : 207 106 kW/m.K)
- Ammonia Boiling point (1.013 bar) : -33.5oC
- Ammonia Latent heat of vaporization(1.013 bar at boiling point):1371.2 kJ/kg
- Ammonia Vapor pressure (at 21oC or 70oF) : 8.88 bar
- Ammonia Critical point - Critical temperature : 132.4oC - Critical pressure : 112.8 bar
- Ammonia Gas Density (1.013 bar at boiling point) : 0.86 kg/m3
- Ammonia Gas Density (1.013 bar and 15oC (59oF)) : 0.73 kg/m3
- Ammonia Gas Compressibility Factor (Z) (the ratio of the actual volume of the gas to the volume determined according to the perfect gas law) (1.013 bar and 15oC (59oF)) : 0.9929
- Ammonia Gas Specific Gravity(air = 1)(1.013 bar & 21oC(70oF)): 0.597
- Ammonia Gas Specific volume (1.013 bar and 21oC (70oF)):1.411 m3/kg
- Ammonia Gas Specific Heat Capacity at constant pressure (cp) (1.013 bar and 15oC (59oF)) : 0.037 kJ/(mol.K)
- Ammonia Gas Specific Heat Capacity at constant volume (cv) (1.013 bar and 15oC (59oF)) : 0.028 kJ/(mol.K)
- Ammonia Gas Ratio of Specific Heats (Gamma: cp/cv) (1.013 bar and 15oC (59oF)) : 1.309623
- Ammonia Gas Dynamic Viscosity(1.013 bar and 0oC (32oF)):0.000098 Poise
- Ammonia Gas Thermal conductivity (1.013 bar and 0oC (32oF)) : 22.19 mW/(m.K)
- Ammonia Gas Solubility in water (1.013 bar and 0oC (32oF)):862 vol/ vol
- Ammonia Gas Auto ignition temperature : 651oC
Ammonia Pressure versus temp ( psig + 1atm = psia)
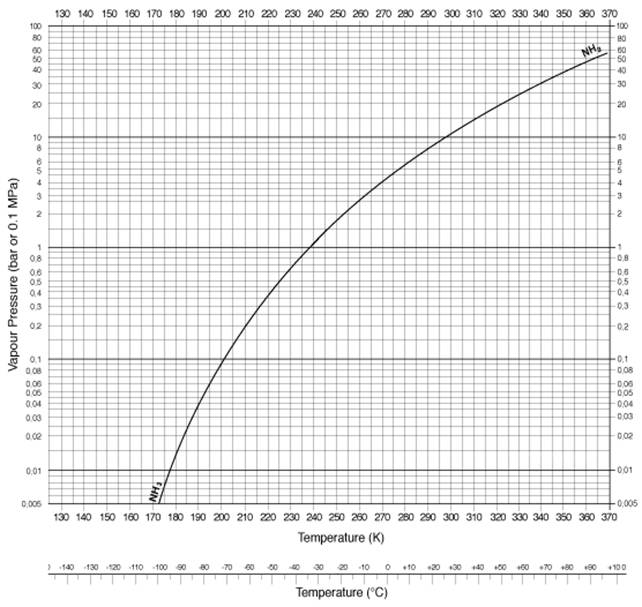
| Temp Kelvin | Temp Celsius | Pressure (bar) | Pressure(psi) |
|---|---|---|---|
| 290 | 17 | 8 | 116 |
| 300 | 27 | 10.1 | 146 |
| 310 | 37 | 15 | 217 |
| 320 | 47 | 18.7 | 271.2 |
| 330 | 57 | 24 | 348 |
| 340 | 67 | 30 | 435 |
| 350 | 77 | 37 | 536.6 |
| 360 | 87 | 47 | 681.6 |
| 370 | 97 | 59 | 855.7 |
Chemical properties(Reactivity & Corrosion Behavior)
- Reaction with air/ oxygen produces nitrogen and water.
NH3 + O2 ➝ N2 + H2 O + Energy - The common metals are not affected by dry ammonia. Moist ammonia will not corrode iron or steel, but it will react rapidly with copper, brass, zinc and many alloys, especially those containing copper. Only steel or ductile iron should be used for ammonia containers, valves, fittings and piping.
- Under normal conditions, ammonia is a very stable compound. It takes excessive temperatures (about 840° to 930°F) to cause it to dissociate slightly at atmospheric pressure. When this happens, the dissociated products are nitrogen and hydrogen.
- Ammonia gas burns in a mixture with air within a limited range. The flammable limits at atmospheric pressure are 15% to 28% by volume of ammonia in air.
- It is poisonous in nature and cause burns on moist skin, irritation in eyes& respiratory tract, and may be dangerous if inhaled in considerable amount.
Basicity
One of the most characteristic properties of ammonia is its Basicity. It combines with acids to form salts; thus with hydrochloric acid it forms ammonium chloride (sal-ammoniac); with nitric acid, ammonium nitrate, etc. However, perfectly dry ammonia will not combine with perfectly dry hydrogen chloride: moisture is necessary to bring about the reaction.
NH3 + HCl → NH4Cl
Acidity
Although ammonia is well-known as a base, it can also act as an extremely weak acid. It is a protic substance and is capable of formation of amides (which contain the NH2− ion), for example lithium and ammonia react to give a solution of lithium amide:
2 Li + 2 NH3 → 2 LiNH2+ H2
Self-dissociation
Like water, ammonia is amphoteric as it react with itself to form its acid and base conjugates:
2 NH3 (l) ![]() NH+4 (aq) + NH−2 (aq)
NH+4 (aq) + NH−2 (aq)
Combustion
The combustion of ammonia to nitrogen and water is exothermic:
4 NH3 + 3 O2 → 2 N2 + 6 H2O (g) (ΔHºr = –1267.20 kJ/mol)
Detection and determination
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of Nessler's solution, which gives a distinct yellow coloration in the presence of the least trace of ammonia or ammonium salts. Its presence can also be felt at 5 ppm concentration by its pungent smell.









